Account Holder? Login for Wholesale Pricing
 Call 1-800-661-9891
Mon-Fri 8am-8pm | Sat 9am-6pm (EST)
Call 1-800-661-9891
Mon-Fri 8am-8pm | Sat 9am-6pm (EST)
Globe COVID-19 Rapid Response Test Kit
No Longer Available
This part is no longer available from the manufacturer.
| SKU: | COV-19C25 |
Key Features
- Rapid Response Test Kit
- Tests for SARS-CoV-2 viral nucleoprotein antigens
- 25 Individual Tests per Box
- Results in 15 Minutes
- Nasal Swab test
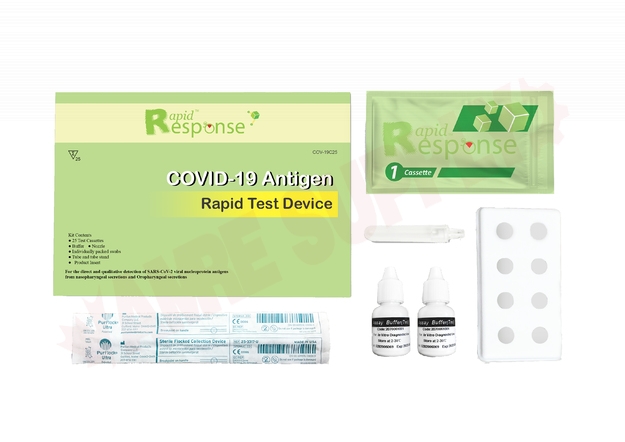
Globe COVID-19 Antigen Rapid Response Test Kit. Able to test 25 individuals per box.
Includes:
Individually packed test devices
Extraction Buffer
Extraction Tube
Nozzle with filter
Tub Stand
Individually packed swabs
Easy to follow instructions
RESULT INTERPRETATION
POSITIVE: Two coloured bands appear on the membrane. One band appears in the control region (C) and another band appears in the test region (T).
NEGATIVE: Only one coloured band appears, in the control region (C). No apparent coloured band appears in the test region (T).
INVALID: Control band fails to appear. Results from any test which has not produced a control band at the specified read time must be discarded. Please review the procedure and repeat with a new test.
These tests are an in vitro immunochromatographic assay for the direct and qualitative detection of SARS-CoV-2 viral nucleoprotein antigens from nasal, nasopharyngeal, or oropharyngeal secretions from individuals suspected of COVID-19 within 6 days of symptom onset. SARS-CoV-2 viral nucleoprotein antigens are generally detectable in nasopharyngeal and nasal secretions during the acute phase of infection. Positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. Negative results should be treated as presumptive, and do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions
Product Downloads
Users Manual
Technical Instructions